Projects
[M.S. Project]
Ligand-capped colloidal metal nanoparticle catalysts
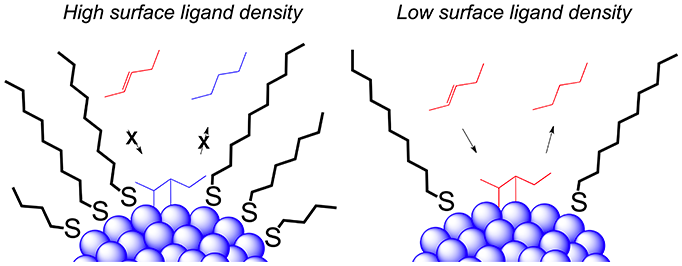
Ligand-capped colloidal metal nanoparticles have several unique properties that make it an intriguing vehicle for catalysis. Although heterogeneous in nature, these catalysts can be dispersed in solution and emulate the catalytic performance of homogeneous catalysts, yet can be easily removed from solution and recycled. The primary role of ligands are to stabilize nanoparticles from aggregation, however, they also have an affect on the activity and selectivity of the organic transformations they catalyze.
In the culmination of this project we developed a novel preparation of these catalysts where we can control the degree of surface ligand density while also sustaining the size of the nanoparticle metal cores. Through this we were able to isolate the effects of surface ligand density on catalytic activity and selectivity independent of core size.
[Ph.D. Projects]
Organic synthesis “on-water”
For certain reactions an enhanced rate and/or shift in product selectivity is observed when performed in a heterogeneous aqueous-organic emulsion. This has been termed the “on-water” effect and has been widely attributed to interfacial hydrogen bonding between water and the substrates/intermediates.
For a certain 1,3-dipolar cycloaddition, we initially observed that performing the reaction “on-water” resulted in a shift in product selectivity. Monitoring of the reaction over time with varying parameters (e.g. agitation, conc., co-solvent, additives) allowed us to optimize this “on-water” effect and form the desired product with high selectivity (<20:1). This study has extended to several other related 1,3-dipolar cycloadditions.

Bioorthogonal proximity-guided “click” chemistry
Photoredox catalysis
Visible-light photoredox catalysis is one of the most highly researched field in chemistry today. This is in part due to the wide variety of organic transformations that can be catalyzed in this fashion. In these reactions, a photosensitizer absorbs visible light and converts it to usable chemical energy (e.g. a single electron reduction/oxidation or energy transfer of/with a substrate, intermediate, or co-catalyst).
There is an ongoing effort in applying this regime of catalysis towards triazole-ring opening reactions.

Publications
[3] Vargas, K. M.; San, K. A.; Shon, Y.-S. Isolated effects of surface ligand density on the catalytic activity and selectivity of palladium nanoparticles. ACS App. Nano Mater., 2019, 2 (11), 7188-8196. [Link]
[2] Vargas, K. M.; Shon, Y.-S. Hybrid lipid-nanoparticle complexes for biomedical applications. J. Mater. Chem. B, 2019, 7, 695-708. (issue cover) [Link]
[1] Chin, M. ; Cisneros, C.; Araiza, S. M.; Vargas, K. M.; Ishihara, K. M.; Tian, F. Rhodamine B degradation by nanosized zeolitic imidazolate framework-8 (ZIF-8). RSC Adv., 2018, 8, 26987-26997. [Link]
